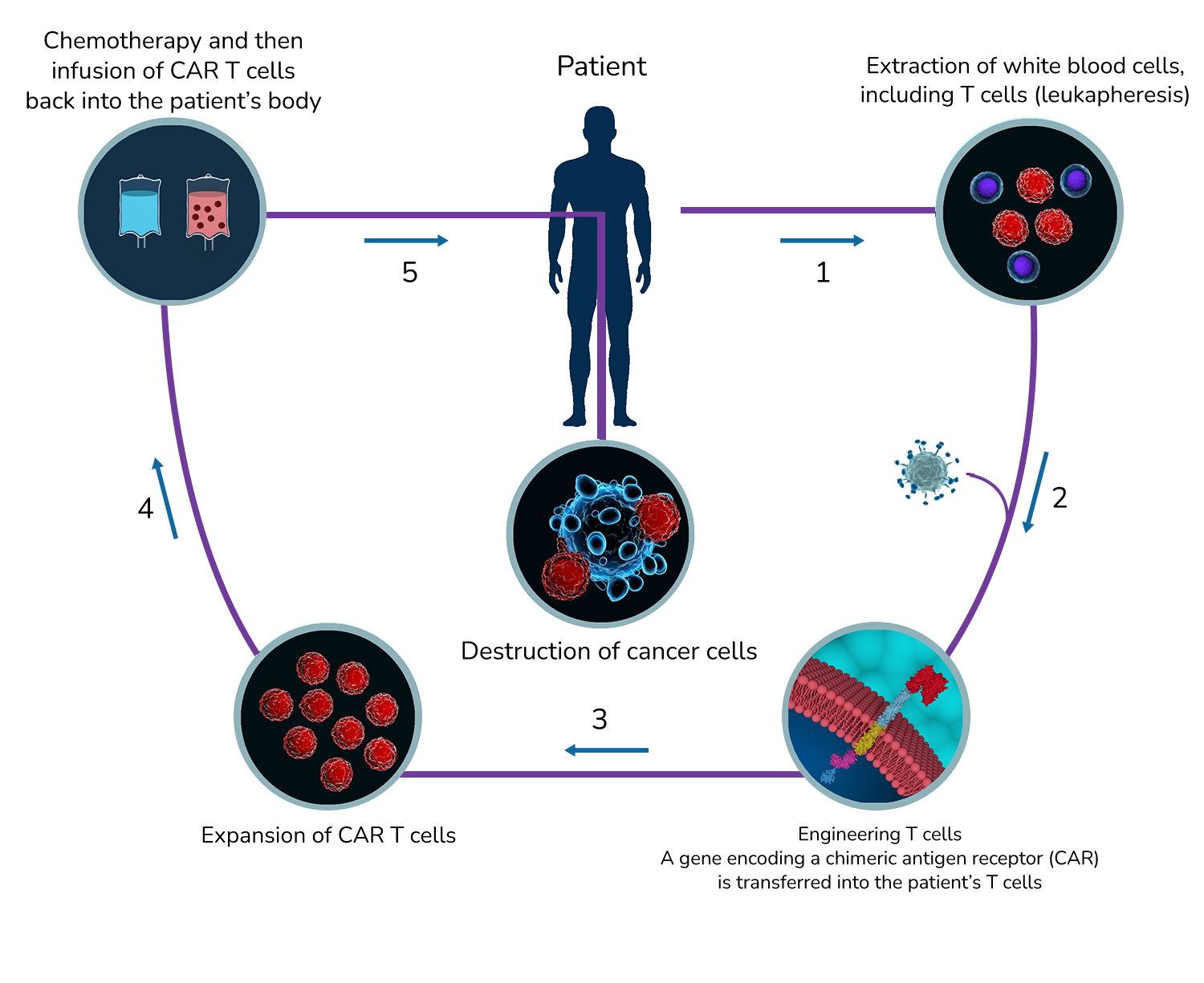
CAR (Chimeric Antigen Receptors) T Cells
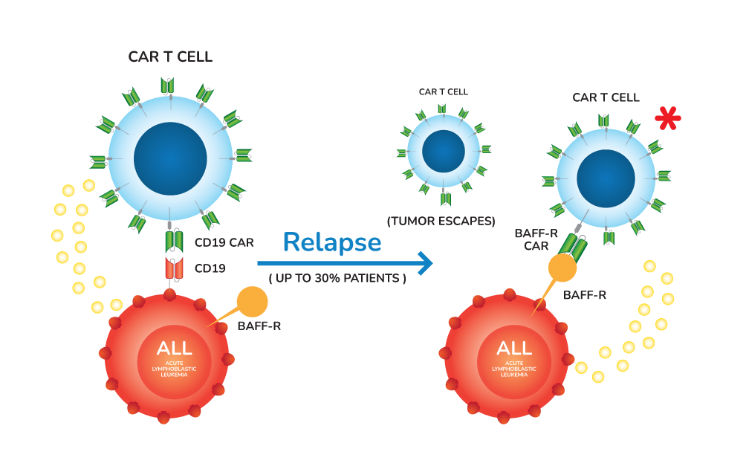
BAFFR CAR-T Cells
Despite high initial efficacy of CD19-CAR T cell treatment for B cell lymphoma and leukemia, some patients relapse through different modes of disease recurrence. Among those relapses post CD19-CAR T cell therapy, 20–30% involves CD19 antigen loss, pointing to the urgent need to identify alternative tumor targets. Since BAFF-R signaling promotes normal B-cell proliferation and appears to be required for B-cell survival, it is unlikely tumor cells could escape immune responses via loss of BAFF-R antigen. This unique characteristic makes BAFF-R CAR T therapy a great potential treatment of B cell malignancies. This hypothesis is supported by the data of our preclinical studies published in Science Translational Medicine
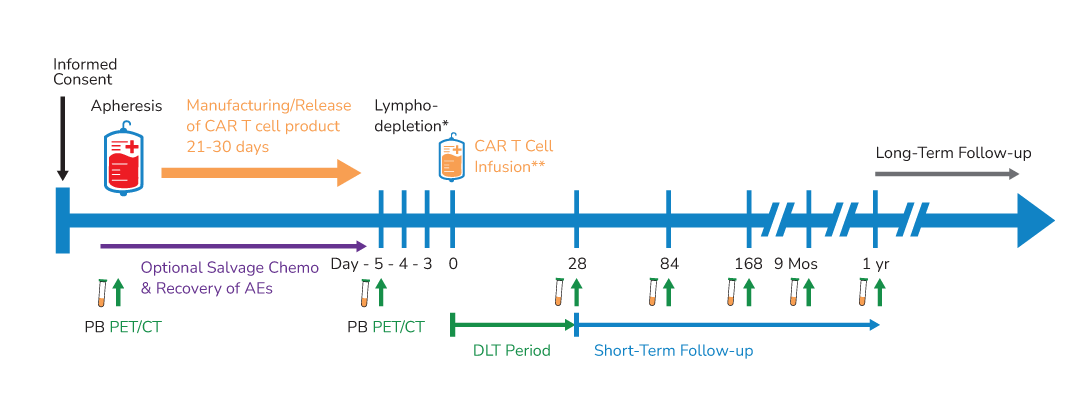
BAFFR T Cells Clinical Trials
PeproMene’s lead candidate, BAFFR CAR-T Cells (PMB-CT01) is currently being investigated to treat relapsed and refractory B-cell acute lymphoblastic leukemia (B-ALL; NCT04690595) and B-cell Non-Hodgkin’s lymphoma (B-NHL; NCT05370430) in phase 1 clinical trials.