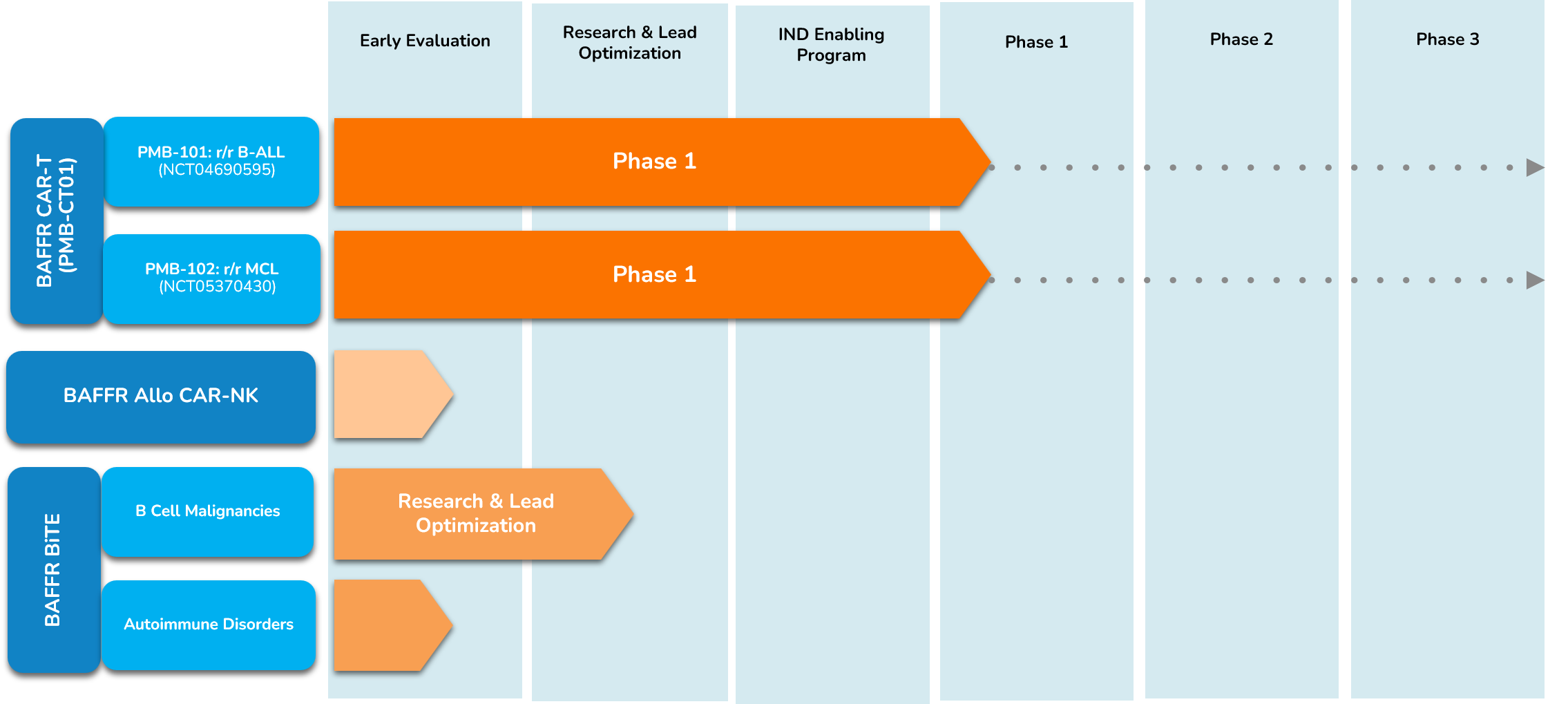
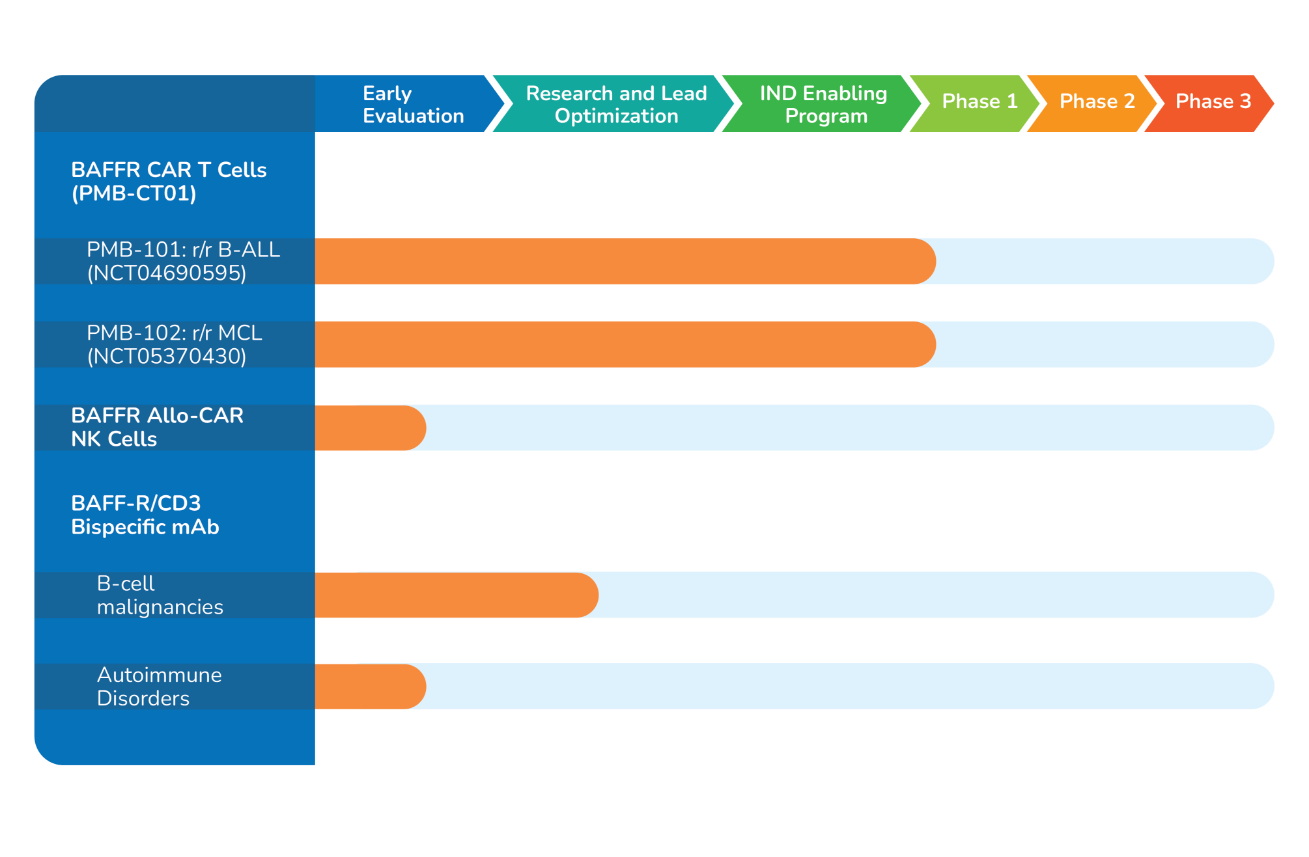
Pipeline
- B-cell malignancies
- Autoimmune Disorders
Pipeline
Expanded Access
PeproMene Bio Inc. is committed to developing safe and effective immune therapies such as BAFFR CAR-T cells to improve the lives of patients with cancer and immune disorders.
At this time, PeproMene does not offer an Expanded Access program. Our lead product, PMB-CT01 (BAFFR-CAR T cells) is investigational, which means that it has not been approved by regulatory health authorities, such as the United States Food and Drug Administration (FDA). While we fully understand that there may be desire to access to our products and recognize the significance of Expanded Access programs, our current priority is to advance our clinical trials as efficiently as possible to ultimately benefit the greatest number of patients. Therefore, PeproMene believes that participation in one of our clinical trials is the best way to access our investigational BAFFR-CAR T therapy. We encourage patients to speak with their physicians regarding participating in the clinical trials. Information on PeproMene’s ongoing clinical trials (NCT05370430 and NCT04690595) can be found in www.clinicaltrials.gov.
As more clinical data on the safety and efficacy of our investigational drugs become available, PeproMene will review and update its policy on Expanded Access. If you have questions about this policy, please contact info@pepromenebio.