CAR NK Cells
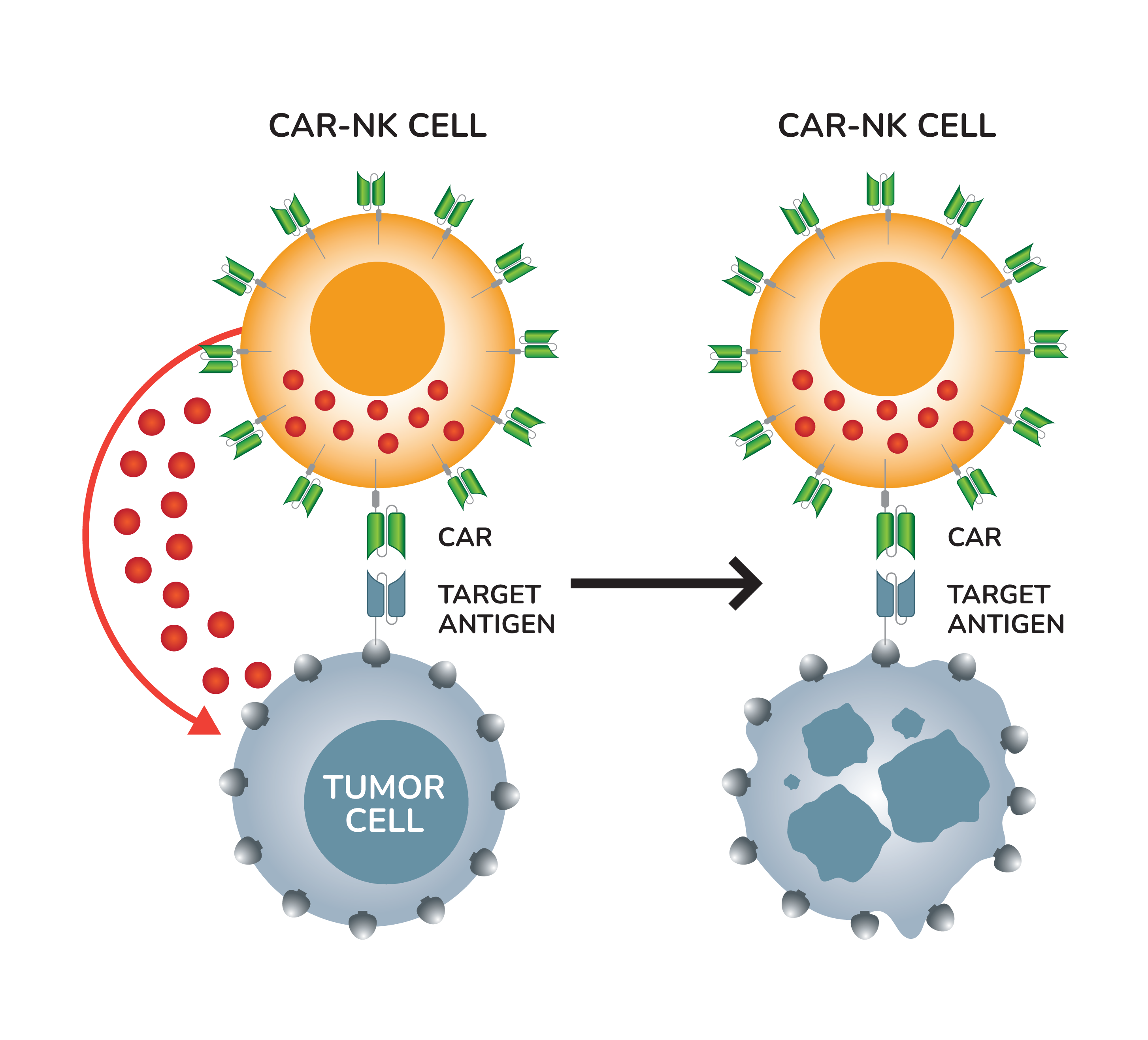
BAFFR Allogeneic CAR NK Cells
BAFFR T Cells Clinical Trials
In sed lorem erat. Sed lacinia auctor ante eget molestie. Proin laoreet neque vel magna dapibus pretium. Nam dictum nisl augue, in imperdiet nisi viverra vel. Suspendisse id tellus sit amet arcu luctus efficitur vel vitae neque. Nam dictum felis placerat, bibendum justo nec, bibendum augue. Nam pellentesque ante ante, elementum consequat ipsum laoreet vel. Integer pulvinar suscipit justo, vitae tempus purus fringilla ac. Aenean luctus iaculis odio vitae facilisis. Aenean bibendum ipsum nec diam tincidunt commodo. Duis in ligula ornare, semper nisl eget, scelerisque ligula.
BAFFR CAR T Cells
Despite high initial efficacy of CD19-CAR T cell treatment for B cell lymphoma and leukemia, some patients relapse through different modes of disease recurrence. Among those relapses post CD19-CAR T cell therapy, 20–30% involves CD19 antigen loss, pointing to the urgent need to identify alternative tumor targets. Since BAFF-R signaling promotes normal B-cell proliferation and appears to be required for B-cell survival, it is unlikely tumor cells could escape immune responses via loss of BAFF-R antigen. This unique characteristic makes BAFF-R CAR T therapy a great potential treatment of B cell malignancies. This hypothesis is supported by the data of our preclinical studies published in Science Translational Medicine.
BAFFR Allogeneic CAR NK Cells
PeproMene is currently assessing the possible partnership with companies having well-established innovative CAR-NK platform to co-develop allogeneic BAFFR CAR-NK therapy.
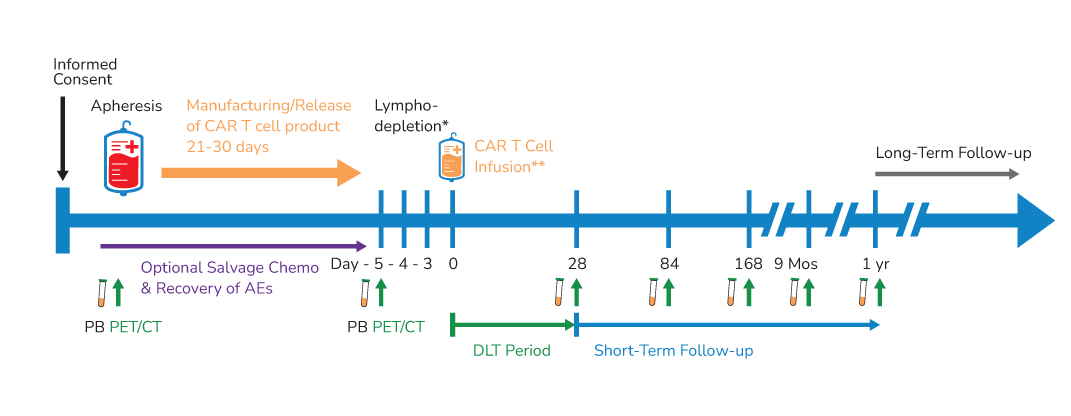
BAFFR CAR T Cells Clinical Trials
PeproMene’s lead candidate, BAFFR CAR-T Cells (PMB-CT01) is currently being investigated to treat relapsed and refractory B-cell acute lymphoblastic leukemia B-ALL; NCT04690595 and mantle cell lymphoma MCL; NCT05370430 in phase 1 clinical trials.

